What is corrosion?
Metals, with the exception of the precious metals such as gold and platinum, that are found in their natural state are always extracted from ores; metals have therefore a tendency to revert to their stable state, which corresponds to their original state, that is to say their oxide form.
Metal corrosion is essentially an electro-chemical reaction at the interface between metal and surrounding environment.
Stainless Steel and the passive layer
Steel is an alloy of iron and carbon. Contrary to carbon steel, the presence of a minimum of 10.5 % chromium in the stainless steel gives it the property of corrosion resistance.
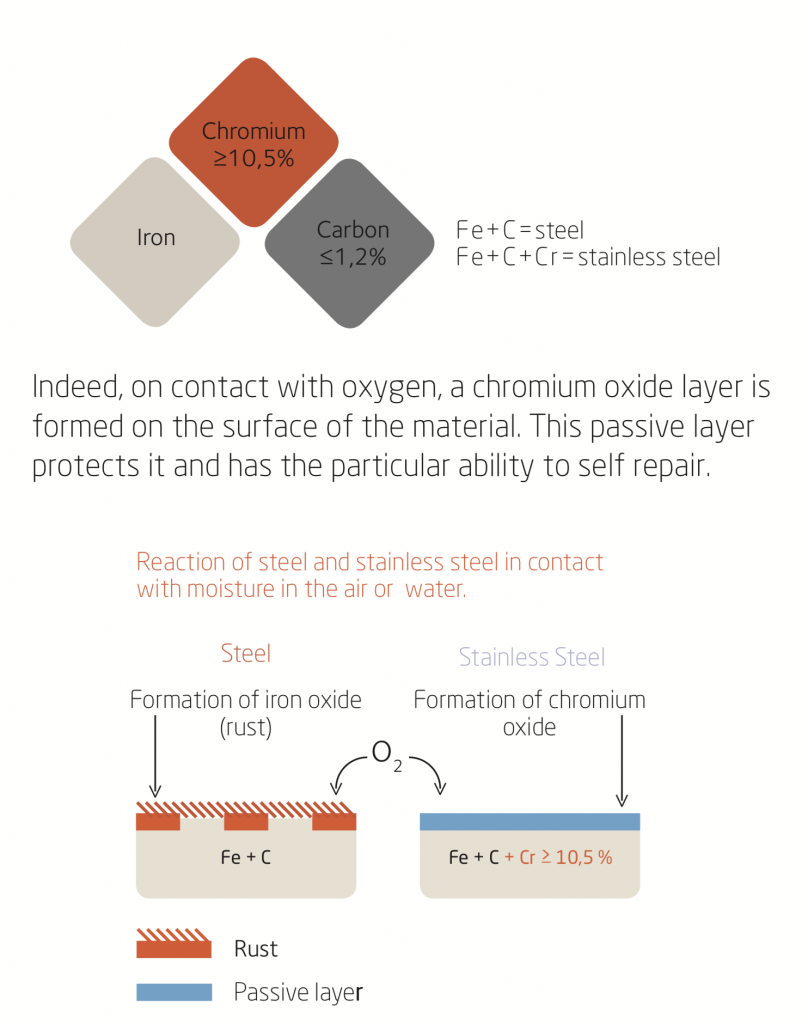
However if this protective layer is damaged, the start of corrosion can appear.
What are the major factors of corrosion?
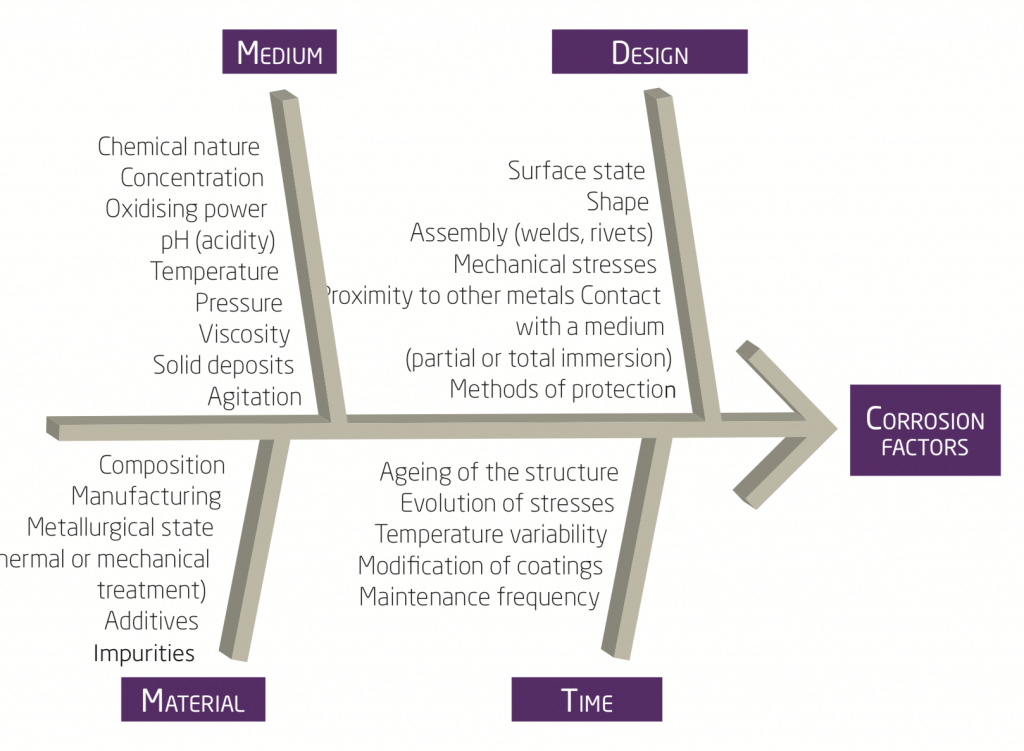
What are the 5 principal types of corrosion linked with the surrounding environment ?
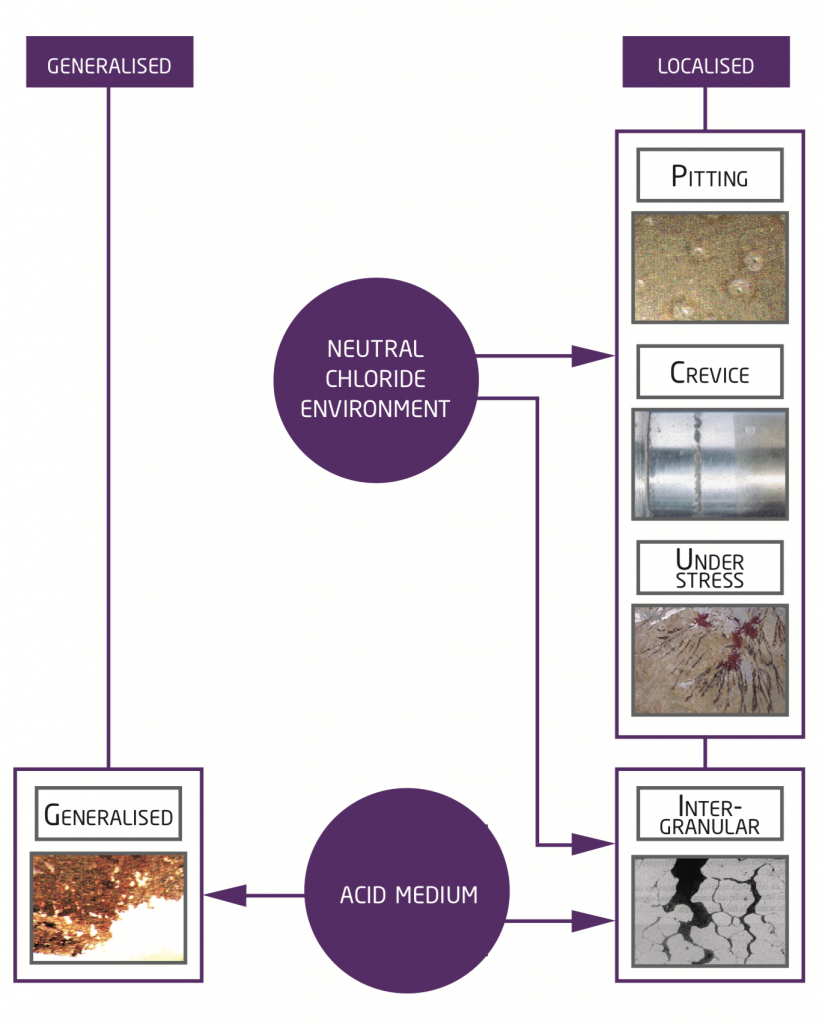
Generalised corrosion is noticed when stainless steel is in contact with an acid medium and localised corrosion is seen in the majority of cases when stainless steel is placed in a neutral chloride environment.
